Welcome to The Gamperl Laboratory

My lab maintains a broad interest in fish and environmental physiology, and marine finfish aquaculture, and takes an integrative multi-level approach to answer research questions in these areas. At present, there are 6 members of my lab, and these staff and students are an active, and integral, part of the research program.
Overview of the Research Program
The Ocean Sciences Centre (OSC) is ideally situated for studying the physiology and biology of coastal North Atlantic species, and provides easy access to fishes that live on the Grand Banks of Newfoundland. At the OSC, I utilize a number of facilities in my research, in addition to my dedicated research labs/spaces, and these include:

1) The Dr. Joe Brown Aquatic Research Building which has state-of-the-art facilities for the rearing and culture of species such as Atlantic cod, Atlantic salmon, lumpfish, wolffish, Halibut etc.

2) The Laboratory for Atlantic Salmon and Climate Change Research (LASSCR) is a self-contained research facility with 10 (2.2 m3) and 9 (0.6 m3) tanks in which temperature, photoperiod and oxygen levels can be controlled / varied. This allows for short-term and long-term studies of fish physiology and immunology at temperatures from below 0oC to > 25oC.

3) The Cold-Ocean and Deep-Sea Research Facility houses disease challenge labs and sophisticated scientific equipment (e.g. deep-sea chambers, Nikon confocal microscope with resonance scanning, Aria III flow cytometer / cell sorter, benchtop scanning EM, fully equipped histological suite etc.). This makes the OSC a great location to study marine fish physiology, immunology and disease processes, and the potential of various fish species for aquaculture production.
In addition, I have recently expanded my research to subtropical/tropical fish species, and have established a research lab on the island of Eleuthera in the Bahamas.
|
Tanks at the Cape Eleuthera Institute |
TMEP-LAB |
|---|---|
|
Harbour Entrance at Cape Eleuthera Institute |
Island School at Sunset |
4) The Tropical Marine Ecophysiology Labratory (TMEP-Lab) was established at the Cape Eleuthera Institute (CEI) of the Island School (Eleuthera, The Bahamas; www.islandschool.org in 2022/2023, as a collaborative effort between CEI and Memorial University. This is a very well equipped facility, and its goals are to:
- Perform long-term measurements of water conditions (e.g., temperature, oxygen, salinity, current velocity, pH) in various marine habitats (e.g., patch reefs, mangrove creeks, and flats etc.) at Cape Eluthera that involves Island School research classes and potentially students from Deep Creek Middle School annually.
- Conduct experiments on various fish (e.g., Nassau grouper, snappers, mojarra, pilchard etc.) and invertebrate (e.g., lobster, crabs, conch, corals) species to understand how climate change-related alterations in environmental parameters (e.g., the increase in global ocean temperatures, hypoxia, heat waves, 'cold-shocks', acidification) may/are affect(ing) the physiology (e.g., stress, metabolism, swimming ability, upper and lower tolerances, growth and performance etc.)
- Work collaboratively with local fishers and communities to understand the impacts of climate change, and to better protect these valuable resources.
In addition, this partnership provides members of my lab with the opportunity to teach research classes in fish physiology in the Island School's semester program.
|
Inaugural Fish Physiology Class Taught at The Island School (Emma Porter Instructor, Middle) |
|---|
My research program focuses on two main themes: 1) the environmental physiology of North Atlantic and Bahamian fish species; and 2) improving culture conditions for, and the health, welfare and disease resistance of, marine finfish cultured in Canada. This research uses a multi-level (telemetry, whole animal, organ/tissue, cellular, genomic etc.) approach to test hypotheses about how environmental conditions (e.g., temperature, oxygen, hydrostatic pressure affect metabolism, swimming performance, cardiovascular function and stress physiology, or how life history and ecology influence the design of physiological systems. In addition, I collaborate with Drs. Matt Rise and Javier Santander (OSC), and Dr. Brian Dixon (University of Waterloo), to investigate how various biotic and abiotic factors affect fish immunology.
Techniques utilized in this research include: the implantation of physiological data loggers and acoustic tags; respirometry for the measurement of fish metabolism/swimming ability; in vivo measurements of cardiovascular function, blood oxygen status and hormone levels; in situ and electrophysiological measurements of heart performance; in vitro measurements of hormone receptors, enzyme activity and kinetics, and vascular function; OROBOROS flourorespirometry for the measurement of mitochondrial and cellular function; and qPCR and RNAseq analyses of gene expression.
|
Sam Heatmap |
Oroboros Fluororespirometers |
|---|

Atlantic Salmon in Swim Tunnel
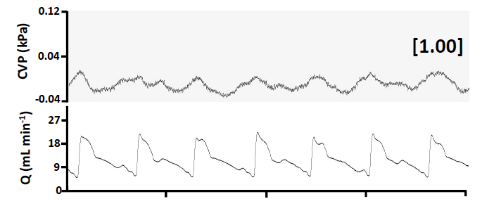
Central Venous Pressure and Cardiac Output in a Nile Tilapia
|
Data Logger Being Inserted into Lumpfish |
Rebeccah Sandrelli at Salmon Cage-Site |
|---|
Examples of Ongoing & Recent Research
Advancing our Understanding of How Cardiovascular Control, Function and Plasticity Influence s Fish Performance and Ecophysiology
We continue to use an integrative approach to examine how metabolism [standard metabolic rate (SMR), max. O2 consumption (MMR) aerobic scope (AS), and mitochondrial function], cardiac function, plasticity and control, and tissue/vascular physiology differ between fish, and how this allows them to exploit their environment. The work is presently being conducted on North Atlantic (e.g., Atlantic salmon; lumpfish, wolffish) and Bahamian species (e.g. Nassau Grouper, schoolmaster snapper, pilchard). A lot of this research focuses on the effects of short-term and long-term changes in water oxygen levels (i.e., hypoxia) and temperature (both warm and cold) on the stress, exercise and cardiorespiratory physiology of fishes, and elucidating the mechanisms that enable fish to survive/function when faced with these challenges. This research’s ultimate goal is to understand the capacity of fishes to respond (both acutely and chronically) and adapt (in the evolutionary sense) to natural and anthropogenic stressors, and the mechanistic basis(es) for these changes.
|
Nassau Grouper at CEI |
Black Grouper in Swim Tunnel |
|---|---|
|
Surgery on School Master Snapper |
Spotted Wolffish in JBARB |
Winter Temperatures and Atlantic Salmon: Physiological Effects and the Mitigation of Negative Impacts
This research, which is an on-going collaboration with Cooke Aquaculture Inc. (Dr. Andrew Swanson), has been extremely successful and revealed that: 1) cardiorespiratory function at cold temperatures, and its response to increased demands, depends on exposure duration; and suggest that aerobic scope does not constrain swimming capacity in salmon when chronically exposed to temperatures approaching their lower limit; 2) that this species stops feeding at 6oC, and experiences sub-lethal stress at 4-5oC and severe stress by 2oC; 3) this species develops behaviours, biochemical imbalances and other clinical signs of a condition termed ‘winter syndrome’ in sea bass and bream that is associated with fatty liver disease (FLD).
At present, we (including collaborators, Drs. Matt Rise, Chris Parrish and Albert Callabres-Solares, and Danny Boyce) are using a multi-faceted approach to examine a number of approaches that could mitigate these two challenges to salmon aquaculture in Newfoundland. This research also involves Dr. Javier Santander, who is identifying pathogens that cause disease at cold temperatures, and is working to develop vaccines against them.
Development of Data Storage and Acoustic Tags for the Measurement of Physiology in Free-Swimming Fishes
This research started with validating the use of Star-Oddi® data loggers (milli-HRT-ACT) that simultaneously record heart rate, activity/swimming speed and body temperature, and using them to monitor free-swimming Atlantic salmon in sea-cages. Since these initial experiments, we have been working with Asgeir Bjarnason and Dr. Felix Mark of Star-Oddi® to develop acoustic versions of these tags, and tags that can measure other physiological variables in free-swimming fishes. These products are close to being commercially available, and will be used to understand the ecophysiology of free-swimming North Atlantic and subtropical / tropical species. Such experimental approaches are important as recent data from our lab suggest that data collected using several commonly used lab-based techniques are not comparable to those measured in fish implanted with data loggers and allowed to recover for extended periods.
MICCSA – Mitigating the Impacts of Climate Change of Salmon Aquaculture
In this pan-Atlantic applied research project involving numerous universities and industrial partners, research tools and products are being developed to: enable the Atlantic salmon aquaculture industry to prepare for, monitor, and predict the effects of warming and hypoxic coastal waters; and to develop fish that are better protected from infectious salmon anemia (ISA) and sea lice (Lepeophtheirus salmonis). These goals will be achieved by:
1. Defining the sub-lethal and lethal temperatures of current Atlantic salmon stocks (i.e., fish of Saint John River origin);
2. Studying how fish with varying genetic background perform under conditions of hypoxia and elevated temperature;
3. Developing molecular markers (single nucleotide polymorphisms, SNPs) for selecting broodstock with high temperature tolerance, robust immune responses and improved disease and stress resistance; and
4. Developing genomic and antibody-based diagnostic assays for assessing salmon health and producing improved, more effective, vaccines.
Research conducted at the Ocean Sciences Center has already: determined the sublethal, and critical thermal and incremental maximal (CTMax and ITMax, respectively) temperatures of Atlantic salmon under normoxia and hypoxia; examined how high temperatures impact fish immunology, and vaccine and antibiotic effectiveness; determined the distribution, behavior and physiology of sea-caged salmon at maximum summer temperatures; identified several key immune- and stress-related genes (biomarkers) that are responsive to temperature / hypoxic challenges; and produced antibodies and ELISAs (Enzyme-Linked Immunosorbent Assays) to several biomarkers so that their protein levels can be quantified and monitored; and determined the hereditability of traits such as ITMax and the fish’s thermal growth coefficient, and identified SNPs that may be useful in selecting temperature-tolerant broodstock.
Collaborators: Dr. Mark Fast (UPEI), Dr. Brian Dixon (University of Waterloo), Dr. Matt Rise (Memorial University), Dr. Roy Danzmann (University of Guelph), Danny Boyce (JBARB Facilities Manager), Dr. Amber Garber and Chris Bridger (Huntsman Marine Science Centre), Debbie Plouffe (Center for Aquaculture Technologies Canada), Jessi Rix (Somru BioScience)





-444x295.jpg)