In Principle:
Genetic Engineering involves the laboratory manipulation of DNA
This may involve isolation of a "gene of interest"
in vivo replication or in vitro "cloning" of the gene
analysis of the cloned gene
gel electrophoresis
nucleic acid transfers "Southern blotting"
DNA sequencing
What does a particular region of DNA do?
Reverse Genetics: DNA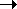 RNA
RNA 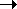 Protein
Protein
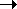 phenotype
phenotype
Bioinformatic comparison with other DNA sequences
Genetic Engineering involves the laboratory manipulation of DNA
This may involve isolation of a "gene of interest"
in vivo replication or in vitro "cloning" of the gene
analysis of the cloned gene
gel electrophoresis
nucleic acid transfers "Southern blotting"
DNA sequencing
What does a particular region of DNA do?
Reverse Genetics: DNA
Bioinformatic comparison with other DNA sequences
Biotechnology: “The use of biological systems to create goods & services"
Restriction enzymes [Nobel Prize 1978]
DNA sequencing [Nobel Prize 1980]
Polymerase Chain Reaction (PCR) [Nobel Prize 1993]
[ For more details, see
Bio4241 - Advanced Genetics
Bio2250 - Principles of Genetics ]
fresh viral, prokaryotic, or eukaryotic material
separation of DNA from protein & lipid with polar / non-polar solvents
"kit" methods: selective binding of DNA
Ancient DNA
museum specimens: 10s ~ 100s years
fossils: 1000s ~ 1,000,000s years
Ex.: Magnolia at 18MYBP
Ex.: insects in amber
Forensic DNA of unknown or questioned origin
blood / semen stains
"The Case of the Falsified Fillets"
"The Case of the Scurrilous Scallops"
"How to Tell a Sea Monster"
DNA Libraries
Expression libraries
mRNA from gene of interest may be abundant in a particular tissue
Ex.: Lysozyme in abomasum stomach of ruminants
Ex.: glue protein in salivary glands of larval Drosophila
mRNA
cDNA will have exons only
Molecular Cloning & Recombinant DNA
Type-II restriction endonucleases
cut DNA only at specific restriction site (expanded list)
DNA palindrome -
"Able was I ere I saw Elba"
"Madam, I'm Adam"
restriction sites read the same in 5'

Overhanging TTAA-5' "sticky ends" can recombine
Vector insertion
Vector - a means of moving DNA from one place to another
Plasmids - a circular, extrachromosomal DNA,
"naked DNA", a "bacterial virus"
pUC18 - an artificial plasmid with:
polylinker containing multiple, unique restriction sites
selectable markers that tell you when plasmid is present
antibiotic resistance (e.g., tetracycline or ampicillin)
lacZ gene produces beta-galactosidase,
metabolizes Xgal sugar
Recombinant DNA molecules are formed when
ligation of "sticky ends" occurs between source DNA & vector DNA
combines genes from two different organisms [online MGA2 animation]
Cloning in E. coli
E. coli K12 strain - The prokaryotic wimp
antibiotic-sensitive
can't metabolize galactose (Xgal) sugars
Host transformation introduces plasmid into bacterial host
Colony Selection: finding the rare bacterium with recombinant DNA
Only E. coli cells with resistant plasmids grow on antibiotic medium
only plasmids with functional lacZ gene can grow on Xgal
lacZ(+) => blue colonies
lacZ functional => polylinker intact => nothing inserted, no clone
lacZ(-) => white colonies
polylinker disrupted => successful insertion & recombination!
Bulk bacterial culture of recombinant white colonies
Bacterial replication also replicates recombinant plasmid
Purify cloned plasmid DNA,
Gene is ready for Analysis
SUMMARY OF pUC CLONING
The Cartoon Guide to Molecular Cloning (Gonick & Wheelis 1991)
In vitro
DNA "cloning": The Polymerase
Chain Reaction
"DNA xeroxing": four components & one gadget
DNA template
anything with DNA in it
oligonucleotide primers
short (20 ~ 30 base) ssDNA complementary to gene
some knowledge of gene is required :
"Universal primers" work across many species
Taq DNA polymerase
heat-stable enzyme from hot-spring bacteria (Thermus aquaticus)
functional at 70 ~ 80oC, withstands repeated exposure to 95oC
dNTPs: four building-blocks for DNA
Thermal cycler: computer-controlled heating & cooling block
PCR doubles gene copy number each cycle
denature / anneal / extend: 2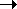 4
4  8
8  16
16  32
32  64 etc.:
64 etc.:
10 cycles = 103 copies, 20 cycles 106 copies,
106 copies,
30 cycles (~2 hrs) 109
copies
109
copies
[click here for an of PCR]
PCR process is completely automated
replicates specific gene only
makes sufficient quantities of purified genes for direct analysis
[mtDNA gene in RFLP Lab amplified by PCR]
"DNA xeroxing": four components & one gadget
DNA template
anything with DNA in it
oligonucleotide primers
short (20 ~ 30 base) ssDNA complementary to gene
some knowledge of gene is required :
"Universal primers" work across many species
Taq DNA polymerase
heat-stable enzyme from hot-spring bacteria (Thermus aquaticus)
functional at 70 ~ 80oC, withstands repeated exposure to 95oC
dNTPs: four building-blocks for DNA
Thermal cycler: computer-controlled heating & cooling block
PCR doubles gene copy number each cycle
denature / anneal / extend: 2
10 cycles = 103 copies, 20 cycles
30 cycles (~2 hrs)
[click here for an of PCR]
PCR process is completely automated
replicates specific gene only
makes sufficient quantities of purified genes for direct analysis
[mtDNA gene in RFLP Lab amplified by PCR]
What are its molecular characteristics?
Restriction mapping
Determining the order of restriction sites in a cloned fragment:
this provides an "outline" of the DNA sequence
Gel electrophoresis separates DNA fragments by molecular weight
DNA is visible under Ultraviolet light with fluorescent dye
(Click here for an of agarose gel electrophoresis)
Fragment sizes in single & pairwise restriction digestions compared:
order & distances among sites determined
(Click here for an of Restriction Mapping logic)
Restriction maps of adjacent fragments assembled as contig map
Southern Blot analysis
Useful when gene of interest is rare: one locus / genome
DNA is transferred ("blotted") to filter paper
Filter is exposed to a DNA probe
Probe - single-stranded DNA, base-complementary to gene of interest
Binds specifically to target DNA immobilized on filter
Radioactively labeled with 32P / 35S-dNTPs
Autoradiogram shows presence / absence & size of cloned DNA
RFLP differences
[click here for an of southern blotting]
DNA probe binds to RNA: "Northern Blot"
Is a particular gene (DNA) expressed as mRNA in a particular tissue?
[Antibody probe binds to protein: "Western Blot"]
DNA sequencing
in vitro DNA replication reaction copies one strand repeatedly
Provides complete order of bases in a DNA fragment
DNA primer is complementary to 3' end of gene of interest
dideoxynucleotide terminators (ddNTPs)
stop strand growth during replication
four separate reactions terminate at ddA, ddC, ddG, or ddT
Sequencing gel shows series of partial DNA replications
Sequencing "ladder" is read from bottom to top
Automated DNA sequencer uses laser fluorometry
ddNTPs are attached to fluorescent dyes
scanning laser & fluorometer "see" fluorescence colours (A C G T)
computer assembles four colours, "calls sequence"
"Helix & Primer" DNA Sequencing Lab does this at MUN
[click here for an of automated DNA sequencing]
Files are in PC or Mac format & require the Shockwave viewer:
Click here to go to the download site