Radiation Mutagenesis & Health Physics
In principle:
Genetic molecules & structures are affected by high-energy
ionizing radiation
ionization may
effect DNA directly or indirectly
Health Physics is the
study & control of the effects of radiation on humans.
Primer of
Ionizing Radiation
isotopes have same atomic number (Z) = # protons
different atomic mass (A) = Z + N (# protons + #
neutrons)
Ex.: 125I
is an isotope of 127I
125 = 53
protons + neutrons (versus 127
A = 53 Z + 74 N) (see periodic
table)
[nuclides: isotopes
differing in energy level:  nucleotides]
nucleotides]
radioisotopes (radionuclides) are unstable:
nucleus
&
electron shell are energetically unbalanced
nucleus undergoes radioactive decay:
spontaneous release of energy and/or mass as particles
or waveforms
Particles
alpha & beta emitters (32P,
35S, 14C, 3H,
131I) [read as "P 32" etc.]
alpha particle: nucleus ejects He
nucleus (2 protons + 2 neutrons)
beta particle: neutron decays to proton + e- (electron)
or,
proton
decays to neutron + e+ (positron)
Waveforms
gamma
emitters
(137Cs)
[Beta-decay to 137Ba,
then] electron capture: proton + e-
 neutron + gamma photon
neutron + gamma photon
Planck's Equation predicts energy content:
E = h / 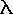 where E
= energy,
where E
= energy, 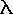 = wavelength, h =
Planck's constant
= wavelength, h =
Planck's constant
shorter wavelength radiation  more energetic radiation
more energetic radiation
Energy: UV (ultraviolet) radiation < X-rays < gamma rays < cosmic rays
Ex.: long-wave UV B
in "black lights" is safer than short-wave UV
A in tanning beds
Neutron
activation: N bombardment renders
materials radioactive
"Criticality Incidents"
&"Nuclear
Excursions"
Louis
Slotin (1910 - 1946), Canadian physicist at Los
Alamos
Los Alamos
(Dec 1958) & Tokaimura (Sept
1999) accidents
Enhanced
Radiation Weapons: "Neutron
Bombs"
"Fallout"
fission & fusion weapons
introduce radioisotopes into the environment &
food chain
Neutron activation of atmospheric elements
Nuclear reactor accidents release
short- & long-lived radioisotopes
Ex: Chernobyl (April 1986) &
Fukushima
Daiichi (March 2011) release 137Cs &
131I

Genetic
effects
of
ionizing radiation
direct
or indirect radiochemical damage to DNA
direct
effects: formation of thymine dimers
(T~T)
covalent
linkage
of adjacent T T bases
causes errors in replication
UV irradiation
causes skin cancer
photoreactivation or excision repair reverse
damage
xeroderma pigmentosum
is a genetic disease
caused by a repair defect
cross-linking - different DNA molecules covalently
joined
H-bonds  covalent bonds
covalent bonds
dsDNA
chromosome
breaks
non-homologues
join
end-to-end to form dicentric
chromosomes
radium watch dial
painters (1920s) ingested 226Ra [high-energy alpha]
indirect effects: oxidative
damage
Radiolysis
of
H20 produces free radicals:
H2O 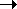 H + OH
[hydroxy radical]
H + OH
[hydroxy radical]
HO + OH 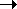 H2O2
[hydrogen peroxide]
H2O2
[hydrogen peroxide]
H2O2
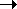 H
+ HO2-
[superoxide radical]
H
+ HO2-
[superoxide radical]
oxidation of bases modifies pairing rules
Ex.: 8-oxo-7-hydro-deoxyguanosine (GO)
dG 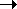 GO by oxidation, pairs with
A
GO by oxidation, pairs with
A  transversion
transversion
Prevention & repair of
oxidative damage
superoxide
dismutase (SOD): HO2- + H 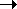 H2O2
H2O2
catalase: H2O2 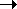 H2O
H2O
Radioisotope exposure & laboratory
safety (Health Physics)
Half-life
( t1/2)
physical - Time to lose 1/2 of radioactivity by
physical decay (Tp)
amount remaining = 0.5t
Ex.:
10 half-lives leaves 1 / 1024 ~ 0.1%
131I:
t1/2 = 8 days
137Cs:
t1/2
= 30.2 yrs
biological - Time to eliminate 1/2 of material
from body metabolically (Tb)
131I:
t1/2 = 138 days
137Cs:
t1/2 =
70 days
effective - combined physical & biological decay
loss ( 1 / Te = 1/ Tp
+ 1/Tb )
then Te
= (Tp)(Tb)/
(Tb
+ Tp)
HOMEWORK: Prove the
formula; calculate Te
for 131I and 137Cs
body burden - Amount of material that stays in body
permanently
critical
organ
depends on isotope
239 Pu - Plutonium: calcium analog, "bone-seeker"
131,125 I - Radioiodine:
used in tests of thyroid function as "thyroid-seeker"
3 H - Tritium:
enters "body water"
Dosimetry
of ionizing radiation
Measures of
mass
curie (Ci) = 1 gm of radium (226Ra) = 3.7 x 1010 dps
1 dps = 1 disintegration per second = 1 becquerel
(Bq)
1 PetaBq (PBq)
= 1015 Bq = 27 kCi [Table of SI Units]
Ex.:
Acute Polonium
(210Po) poisoning as assassination tool
Measures of
dose
How
much
radiation strikes target?
Measure
this
with a Geiger-Muller tube ("Geiger counter")
1 Gray
(Gy) = 100 Roentgen (R) = 1 J / kg
Ex.:
typical X-Ray series =
2.2 mGy = 220 mR
Public Health concerns arise from large-scale release
Ex.: Hiroshima (Aug
1945) detonation (14 kt TNT) released 59 x 1012
J (59 TJ)
= 8 ×1024 Bq =
8 YottaBq
Ex.:
TMI-2
accident (March
1979) released ~500 PBq (13 MCi)
135Xe
& ~ 0 Ci 137Cs
& < 20 Ci 131I
Ex: Chernobyl
accident (April 1986) released ~85 PBq (2.3 MCi)
137Cs & 1,760 PBq
(47.5
MCi) 131I
Environmental
Contamination < 1500 kBq / m2
Exposure of Reactor crew
Ex: Fukushima
Daiichi accident (March 2011) released ~15 PBq
137Cs & 500 PBq
131I
Measures of effect
How much radiation is absorbed
by body ?
Depends on radiation type & target
1 Gy
X-Rays delivers 100 rad (radiation-absorbed
dose)
Biological effect depends
on
nature
of radiation
1 Gy X-Rays delivers 1 Sievert (Sv)
or,
10mSv
= 1 rem [roentgen-equivalent
(in)
man - effect dose]
Depends on relative biological effect
(rbe) of radiation
e.g., radionucleotides as nucleic acid labels
(32P- & 35S- dNTPs)
exposure  little effect [except: risk of cataracts]
little effect [except: risk of cataracts]
ingestion  direct
incorporation
into chromosomal DNA
direct
incorporation
into chromosomal DNA  high rbe
high rbe
Linear Energy Transfer (LET)
= energy / path length
How much energy is released during passage through cell
/ body?
gamma & X-rays: (very) high energy over long
path length  low LET
low LET
alpha & beta particles: low energy over (very)
short path length  high LET
high LET
high specific ionization over short
path produces intra-cellular damage
Consequences of radiation exposure
Average North American exposed to ~6.2 millisievert (6.2 mSv) = 620 mrem
/ year)
LNT (Linear No Threshold) model
assumed but challenged below ~ 1 mSv
Dose-response curve is
linear: no safe threshold
[HOMEWORK]
Acute high-level
exposure induces genetic mutation
Chronic
low-level
exposure increases individual risk & population incidence
of cancer
100 mSv increases individual
lifetime risk of death from cancer by 0.4%
Ex.: Post-Chernobyl
contamination: >1 Ci 137Cs / km2
 added dose ~1 mSv / yr
added dose ~1 mSv / yr
US & Canadian NRC sets occupational
limits to exposure
Exposure threshold
for civil population emergency = 10 ~ 50 mSv (Fukushima 2011)
Acute exposure to 500
mSv produces 'haematopoetic syndrome' =
"radiation
sickness"
3,000 mSv produces 'gastrointestinal syndrome',
fatal without treatment
5,000 mSv = LD50/30
: lethal dose for 50% of
population in 30 days, even with treatment
Homework: Consider
10 uCi of a gamma- versus an alpha-emitter
Which would
you rather be exposed to
at 1 meter?
Which would you rather swallow?
Explain.
 neutron + gamma photon
neutron + gamma photon 

 covalent bonds
covalent bonds little effect [except: risk of cataracts]
little effect [except: risk of cataracts]