Non-Standard Base
Pairing of rare tautomers
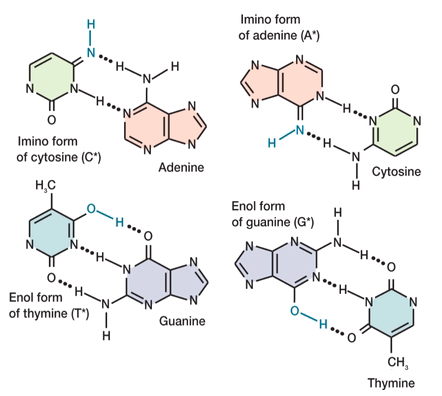
Specificity of base pairing between two-ring purines and one-ring pyrimidines is determined by the number of hydrogen (H) bonds available. Recall that the standard forms of C and G pair with three H-bonds, and the standard forms of A and T pair with two.
The rare tautomeric form C* has only two H-bonds, and pairs with the alternative purine, A [above, left]. Likewise, the rare tautomeric form T* has three H-bonds, and pairs with the alternative pyrimidine, G [below, left].
In contrast, the rare tautomeric form G* retains three H-bonds, but the tautomeric arrangement of bonds forces them on the alternative pyrimidine, T [below, right]. The rare tautomeric form A* retains two H-bonds, and forces them on the alternative pyrimidine, C [above, right].
In summary, the rare tautomers pair with the alternative ("wrong") purine or pyrimidine: C* with A, T* with G, G* with T, and A* with C, respectively. This altered pairing preserves the three-ring structure of the base pairs.
Figures & Text ©2024 by Steven M. Carr