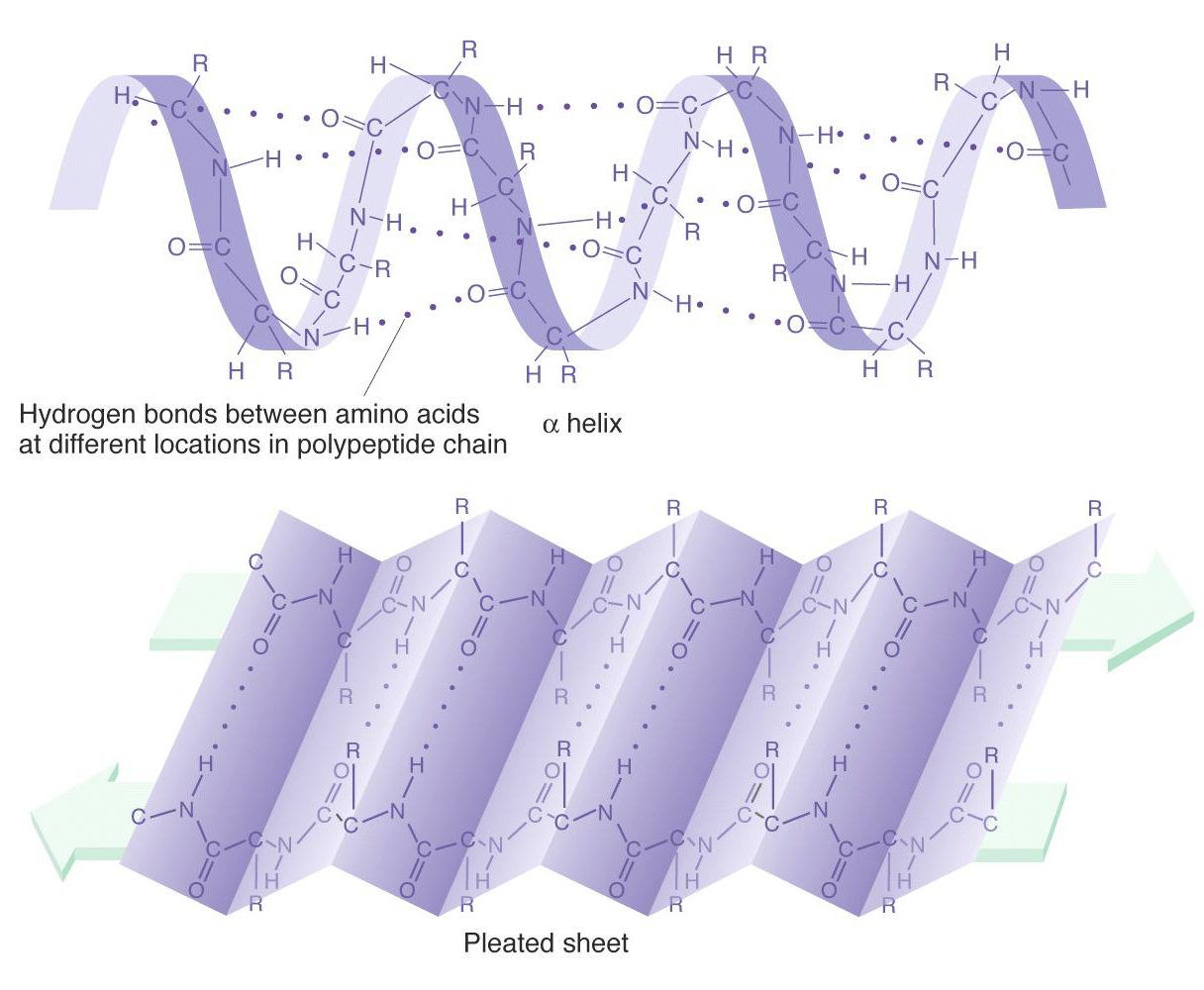
Secondary Protein Structure:
Alpha helices & Beta pleated sheets
The alpha helix
and beta
pleated sheet forms are alternative configurations of the N - C(R) - C backbone residues. The alpha helix is a right-handed helix,
from which the radical (R)
groups are directed outward at right angles. The helix is stabilized by
H-bonds between non-adjacent residues. The beta sheet consists of a series of
molecules
running anti-parallel to each other. The
radical (R) groups are directed
outward at right-angles, alternatively above and below the plane of the
sheet: the sheet is stabilized by H-bonds between carboxy and amino
groups of adjacent antiparallel molecules.
These regular structures are a consequence of the exclusively L forms of the
amino acids.
Figure © 2002 by Griffiths et al. ; All text material ©2010 by Steven M. Carr