Quatenary
Structure
Some
enzyme proteins are made up of two or more subunits
Monomeric proteins – one subunit
Heterozygotes show two bands,
one for each allozyme.
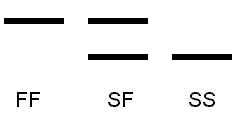
Dimeric proteins – two subunits
Heterozygotes shows three bands
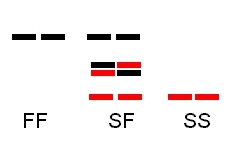
Gels:
1.
Monomeric enzyme protein (PGM): two heterozygotes FS and MS:
2.
Dimeric enzyme protein (PGI): SF, SS, SF

3. Triploid pattern (3 allozymes: S, M, F) in a monomeric

Exercise:
Use ImageJ to
quantify variation in banding intensity among
Click HERE to start the exercise.
From a lab exercise orginally prepared ©2005 by DJ Innes; text ©2008 by Steven M. Carr