
In vivo formation of a Peptide
Bond & growth of the polypeptide chain
The
amino
acids in the ribosome are
attached
to
their respective tRNAs by
an
ester bond (R - O - R) between the carboxyl terminus and the
amino acceptor stem
(left). During formation of a peptide bond, the ester bond in the (P)eptidyl site is
cleaved, and Peptidyl
Transferase catalyzes a condensation
reaction between the carboxyl
terminus and the amino terminus of the amino acids in P and A sites.This
transfers the P-site
amino acid to the A-site
amino acid, and the
original amino terminus remains unmodified.
The
polypeptide thus "grows" N 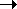 C from the amino
terminus to the carboxyl terminus.
C from the amino
terminus to the carboxyl terminus.
In vitro formation of a peptide bond is a dehydration reaction that splits out of an H20. However, the in vivo reaction is a condensation reaction. Because the C-terminus of the amino acid in the P site is joined to the 3' terminus of the tRNA by an ester bond, it participates in peptide bond formation as a carbonyl radical (-C=O) without an -OH radical. When the C-terminus joins with the NH2 terminus of the amino acid in the A site, the end result is the shift of a proton (-H) from the amino terminus to the uncharged tRNA in the P site. This balances the reaction, and forms a peptide bond without release of an H20 molecule. The in vivo rxn is self-contained.
In vitro formation of a peptide bond is a dehydration reaction that splits out of an H20. However, the in vivo reaction is a condensation reaction. Because the C-terminus of the amino acid in the P site is joined to the 3' terminus of the tRNA by an ester bond, it participates in peptide bond formation as a carbonyl radical (-C=O) without an -OH radical. When the C-terminus joins with the NH2 terminus of the amino acid in the A site, the end result is the shift of a proton (-H) from the amino terminus to the uncharged tRNA in the P site. This balances the reaction, and forms a peptide bond without release of an H20 molecule. The in vivo rxn is self-contained.
Figure corrected from
©2010 PJ Russell, iGenetics 3rd ed.; all text material ©2024 by Steven M. Carr